
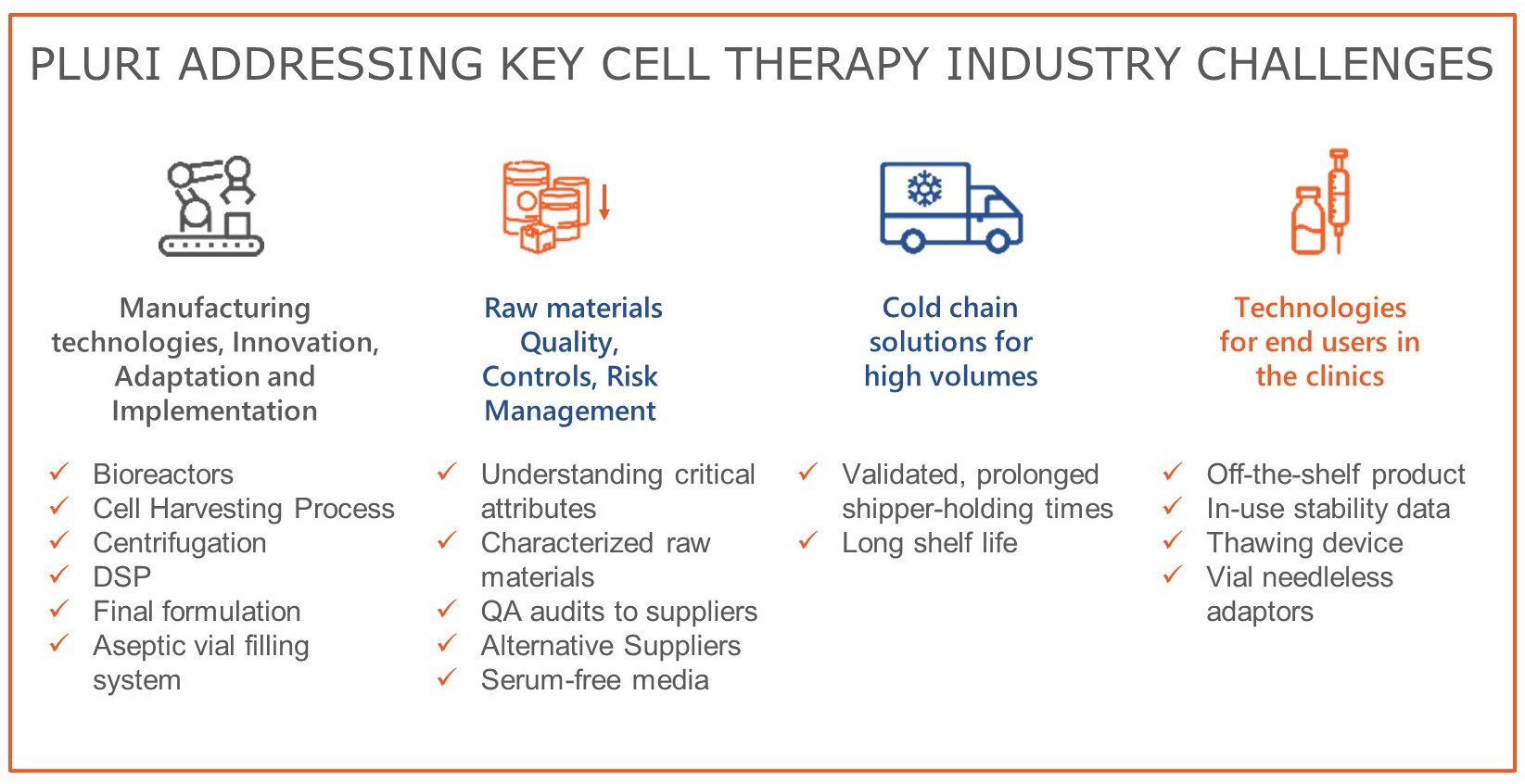
Innovative technology underpins our service and partnership strategy. Through more than 20 years developing cell-based therapeutics, Pluri has encountered the key challenges facing cell therapy manufacturing such as scaleability, cell expansion, recovery and processing, and has developed innovative technological solutions which have been implemented in our laboratories and operated under GMP conditions in production facilities. Pluri holds over 250 patents covering many aspects of culture and manufacture of stem cells and stem cell-based products. Our overall strategy has been to develop automated, closed system platforms that can be scaled from bench-top operation in development to large scale in commercial GMP. We offer proven, reliable processes, with low batch to batch variability, that have been operated in GMP with the approval of major regulatory agencies.
Traditional methods for culture of adherent cells such as stem cells are difficult to scale. Pluri has developed a novel approach, based on a fixed bed of non-woven proprietary polymer matrix, which can be utilised in a conventional stirred tank bioreactor system in perfusion, to provide a high performance and high-density platform for the culture of adherent stem cells. Pluri’s fully automated, closed system, is scalable from 400ml to 14L, and can produce batches of over 4×1010 cells in GMP.
The polymer matrix macro carrier is 10x more efficient than micro carrier systems in stirred tank bioreactors and is adaptable across a variety of cell types, including MSC, iOSC, HEK293, CHO and even plant cells.
Operating in perfusion culture, the system is ideally suited for harvesting EVs and Secretome.

In general, larger batches that provide more doses per batch typically represent a more efficient use of facilities and time, and result in lower costs per dose.
Pluri is committed to developing scaleable production platforms that can be operated to GMP in a commercial-scale manufacturing context, so as to enable to efficient production of cell-based products at viable costs-of-goods.
Pluri has scaled out the bioreactor system allowing us to produce 35L batches with over 130x 10e9 cells per batch. The system is fully automated and fully closed, maintaining quality and assurance of batch-to-batch reproducibility.
Harvesting adherent cells from culture systems after expansion can be challenging, and it is important to minimise cell losses and cell damage, whilst at the same time releasing cells from attachment with high efficiency. Pluri has developed a system for doing this, a proprietary combination of enzymatic reaction and mechanical forces. Pluri’s platform is a 21CRF part 11 compliant, fully closed system which can recover cells with >90% efficiency.
Our downstream process development has been consistent with our overall philosophy, focusing on scalability, automation, closed systems and batch to batch consistency. We use continuous, automated, closed centrifugation for washing and formulating cells. Our platform can be scaled out and is capable of handling large batches of cells. We use a closed and scalable formulation system and automated and closed vial filling platform.
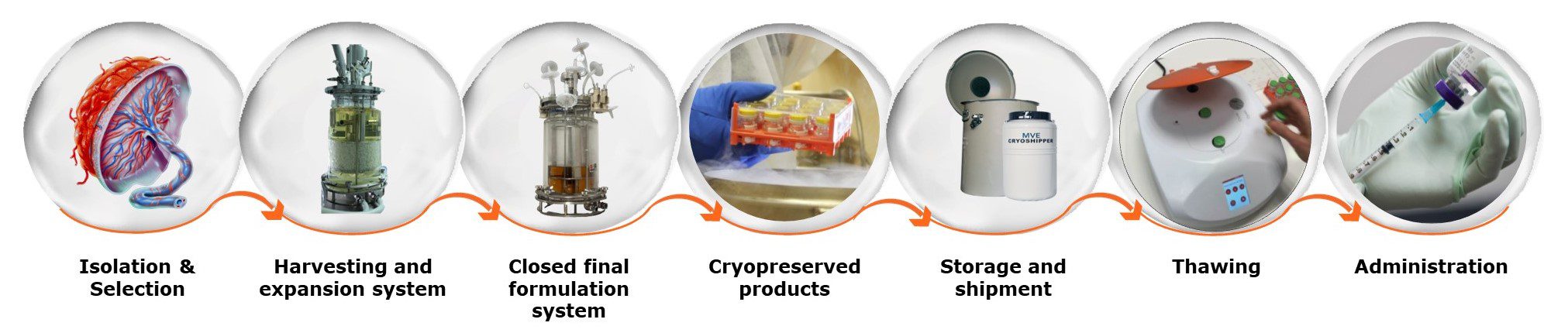
Pluri has manufactured products that have been used in clinical trials in Europe, USA, S. Korea and Japan. Recognizing that our products are living cells, we have invested significant resource and expertise in cryopreservation, storage, shipping and recovery of living cells, to ensure that product quality is preserved to the maximum extent from manufacture to patient.
©2023 Pluri™ Inc., its logo, brand, product, and process names appearing in this issue are the trademarks of Pluri™ Inc. or its affiliated companies. All other brand, product, and process names appearing are the trademarks of their respective holders. Reference to or use of a product, or process other than those of Pluri™ Inc. does not imply recommendation, approval, affiliation, or sponsorship of that product, or process by Pluri™ Inc. Nothing contained herein shall be construed as conferring by implication, estoppel, or otherwise any license or right under any patent, copyright, trademark, or other intellectual property right of Pluri™ Inc. or any third party, except as expressly granted herein. All information herein is for general information only and is subject for change without notice.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.