

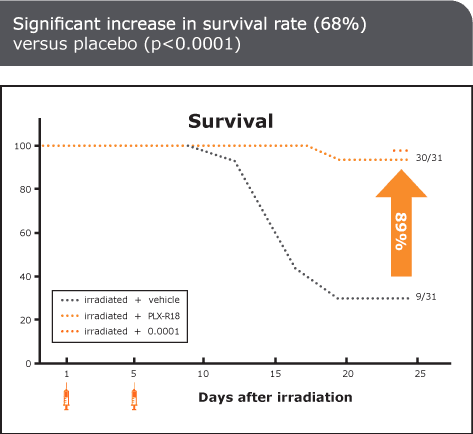
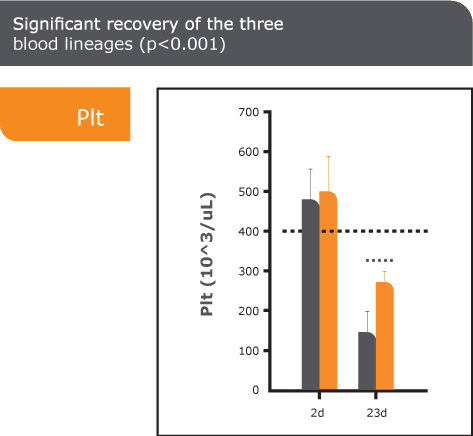
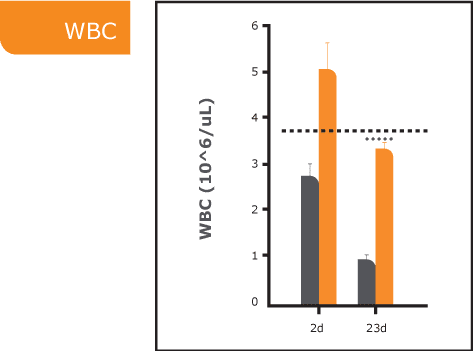
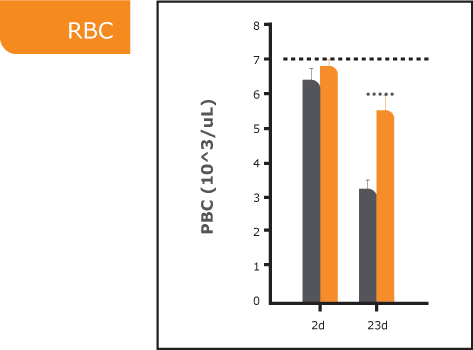
ARS, also known as radiation sickness, occurs after exposure to high doses of ionizing radiation, such as in nuclear accidents or warfare. It leads to severe damage to the bone marrow, impairing the body’s ability to produce blood cells and causing life-threatening complications. Without effective treatment, ARS can be fatal. Currently approved treatments primarily promote white blood cell regeneration and provide only partial relief from ARS symptoms. There is a significant need for new medical countermeasures to improve survival outcomes by addressing all three blood lines affected by ARS.
PLX-R18 is an advanced placenta-based cell therapy designed to stimulate bone marrow regeneration and restore the production of all blood cell types following radiation exposure. It is currently being evaluated as both a treatment for ARS and a prophylactic countermeasure to protect against radiation-induced damage.
Through our collaborations with leading U.S. government agencies, Pluri is committed to developing PLX-R18 as a critical countermeasure for ARS. Our research continues to validate its safety, efficacy, and potential to save lives in the event of radiological or nuclear emergencies.
Pluri also announced it has signed an exclusive collaboration agreement with Hemafund, a Ukrainian umbilical cord blood bank, to establish a strategic initiative for stockpiling, distribution, and potential clinical advancement of PLX-R18 as a countermeasure against Hematopoietic Acute Radiation Syndrome in Ukraine.
For more information on PLX-R18 and our research, contact us today.
Let’s Work Together
Contact us today to explore a partnership tailored to your current and future clinical drug product manufacturing needs.
©2023 Pluri™ Inc., its logo, brand, product, and process names appearing in this issue are the trademarks of Pluri™ Inc. or its affiliated companies. All other brand, product, and process names appearing are the trademarks of their respective holders. Reference to or use of a product, or process other than those of Pluri™ Inc. does not imply recommendation, approval, affiliation, or sponsorship of that product, or process by Pluri™ Inc. Nothing contained herein shall be construed as conferring by implication, estoppel, or otherwise any license or right under any patent, copyright, trademark, or other intellectual property right of Pluri™ Inc. or any third party, except as expressly granted herein. All information herein is for general information only and is subject for change without notice.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.