

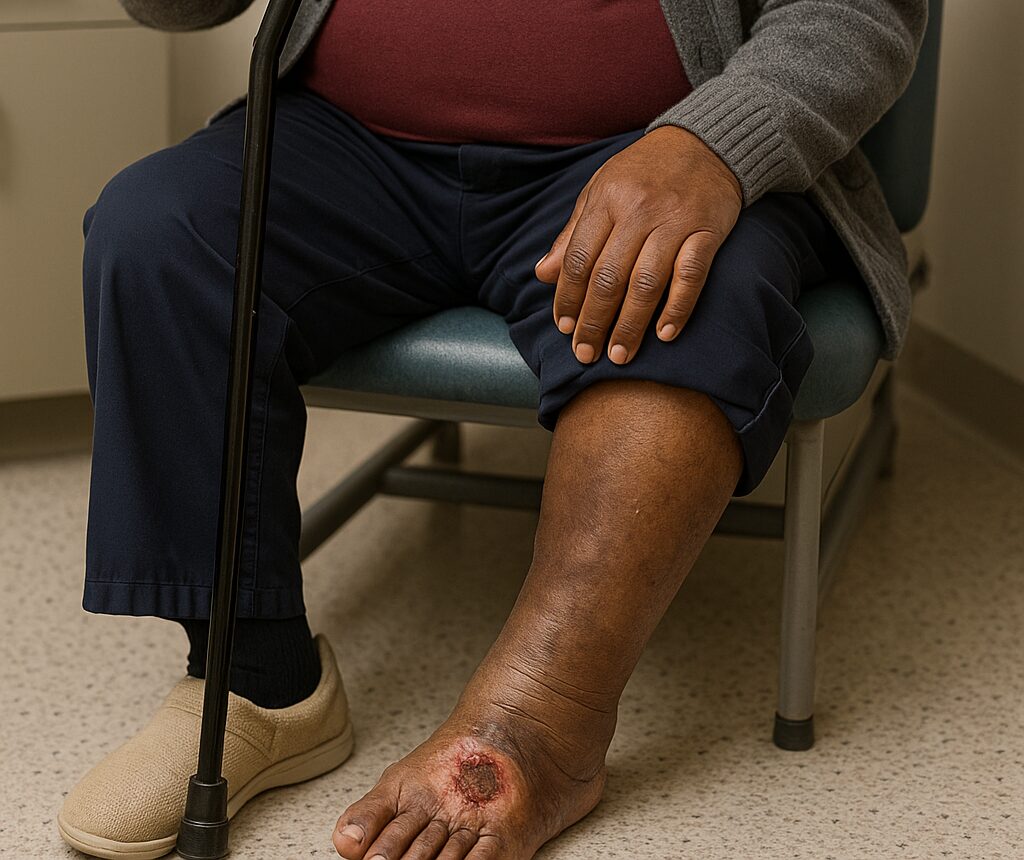
Chronic Limb-Threatening Ischemia (CLTI) is an advanced stage of Peripheral Artery Disease (PAD), caused by fatty deposits in leg arteries that obstruct blood flow. CLTI is affecting millions of patients worldwide, suffering from severe pain at rest, skin wounds, tissue necrosis and poor quality of life with a high risk of leg amputation and death. Up to 35% of patients are unsuitable for revascularization, the standard and only treatment option for severe cases, and they still experience up to a 40% amputation rate at 1 year, with mortality rate of 44% to 85% within 1 to 5 years. Currently, there is no treatment fora significant portion of the population who are considered as “no-option patients”. Those are late-stage CLTI patients (Rutherford class 5= R5) who are unable to go through revascularization or will not benefit from such procedure.
PLX-PAD is an innovative placenta-based cell therapy aiming to treat Chronic Limb-Threatening Ischemia, by secreting a combination of cytokines leading to reduction of inflammation, increasing angiogenesis and stimulating the growth of blood vessels, and reduction of oxidative stress. Efficacy was shown in non-diabetic or well-controlled diabetic patients. By addressing the underlying biological mechanisms of CLTI, PLX-PAD offers a potential treatment where there is a lack of suitable non-surgical therapeutic options for the growing population of patients.
PLX-PAD cells possess regenerative and immunomodulatory properties that are activated in response to ischemia, inflammation and tissue damage. Upon local injection, the cells secrete a combination of therapeutic factors that support the body’s natural repair mechanisms.
These cells promote growth of additional blood vessels to bring oxygenated blood to ischemic tissue, leading to regeneration of damaged muscle and less inflammation. Furthermore, the cells modulate the immune system, which plays a central role in the body’s response to injuries.

PLX-PAD cell therapy has been administered to hundreds of patients globally and is safe and well tolerated.
In a global Phase III study evaluating PLX-PAD as treatment for R5 CLTI in patients unsuitable for revascularization (n=213), significant efficacy was found in a post-hoc analysis for subgroup of non-diabetic or well-controlled diabetic patients with PLX-PAD showed a statistically significant reduction in events of amputation and death at 1-year (p=0.048, HR= 0.46). Notably, the study did not meet its primary endpoint as was defined for the entire R5 CLTI patient group.
This Phase III study, the PACE study, was supported with € 7.6 million grant from the EU Horizon 2020 program.
Learn More
Contact us today to learn more about PLX-PAD for CLTI
©2023 Pluri™ Inc., its logo, brand, product, and process names appearing in this issue are the trademarks of Pluri™ Inc. or its affiliated companies. All other brand, product, and process names appearing are the trademarks of their respective holders. Reference to or use of a product, or process other than those of Pluri™ Inc. does not imply recommendation, approval, affiliation, or sponsorship of that product, or process by Pluri™ Inc. Nothing contained herein shall be construed as conferring by implication, estoppel, or otherwise any license or right under any patent, copyright, trademark, or other intellectual property right of Pluri™ Inc. or any third party, except as expressly granted herein. All information herein is for general information only and is subject for change without notice.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.